Past Issues
An Atypical Presentation of Giant Meckel’s Diverticulitis: A Case Report
Leo Feinberg1, Charlotte Morris2, Ananth Srinivasan1, Pratik Bhattachyra1, K Charan2,Shafquat Zaman1*
1Sandwell and West Birmingham Hospitals NHS Trust, Sandwell General Hospital, West Midlands, United Kingdom 2George Eliot NHS Trust, College Street, Nuneaton, Warwickshire, United Kingdom
*Corresponding author: Shafquat Zaman
Sandwell and West Birmingham Hospitals NHS Trust, Sandwell General Hospital, West Bromwich, West Midlands, B71 4HJ, United Kingdom
Received: November 19, 2019 Published: December 13, 2019
ABSTRACT
Meckel’s Diverticulum (MD) is the most common congenital malformation of the gastrointestinal tract and the only true diverticulum of the small bowel, resulting from the incomplete obliteration of the vitelline duct in the first 5 to 7 weeks of gestation. However, MD is rare, with a traditional prevalence of 2% in the general population. While the majority of MD never become symptomatic, potential for severe complications may arise secondary to diverticulitis with or without perforation, haemorrhage and obstruction. Considerable debate therefore exists whether or not to surgically resect MD found incidentally. Moreover, such complications present considerable diagnostic challenges, and given its rarity, are scarcely considered in the differentials of an acute abdomen.
We present one of the longest cases of giant MD reported in the literature, with non-perforated diverticulitis in a young adult male attending with an acute abdomen and normal inflammatory markers. He underwent successful, un-complicated laparoscopic resection. Histopathological analysis confirmed Meckel’s diverticulitis in the absence of ectopic gastric or pancreatic tissue.
Keywords: Meckels diverticulum; Congential malformations; Laparoscopy; Gastro-intestinal tract
INTRODUCTION
Meckel’s diverticulum (MD) is the most common congenital abnormality of the gastrointestinal tract [1,2]. First reported by Johann Meckel (1809), it arises from the incomplete involution of the omphalomesenteric duct in the 7th week of gestation [1,3,4], and is the only true diverticulum of the small intestine [5].
The maxim of twos is well established in traditional surgical teaching of MD: typically 2 inches long, 2 feet proximal to the ileocaecal valve, present in 2% of the population, twice as common in males and most often symptomatic in patients aged less than 2 years.
Recent evidence has shown a prevalence between 0.3-2.9% of the population [6-13]. Moreover, the largest systematic review of recent literature calculated a weighted mean distance of 52.3cm (7 to 200) from the ileocaecal junction on the antimesenteric border of the ileum, and a mean weighted length of 3.05cm (0.4 to 11), approximatley 1 inch, with a mean diameter of 1.58cm (0.3 to 7) [2].
Giant MD, considered greater than 2 inches (or 5cm), considerably increases the risk of more severe forms of complications, particularly intestinal obstruction [14,15]. Increasing evidence also suggests a further correlation between size and severity of symptoms [2,16].
To our knowledge, herein we present one of the largest cases of MD reported in the literature, with implications for definitive surgical management.
CASE REPORT
A 23-year old Caucasian male presented to the acute surgical take with a short history (several hours) of generalised abdominal pain and one episode of vomiting.
Past medical history included laparoscopic appendicectomy in 2015 with histology revealing a neuroendocrine tumour. The patient was subsequently lost to follow-up. He was otherwise fit and well, on no regular medications and with no known allergies.
On examination his observations were within normal limits and he was maximally tender in the right iliac fossa. Blood results were borderline normal, with a mild leukocytosis (white cell count 12.0 * 10/L) and C-Reactive Protein (CRP) <3mg/L. Urea and electrolytes, liver function tests and serum amylase were within the normal range. Plain radiographs of the chest and abdomen were unremarkable.
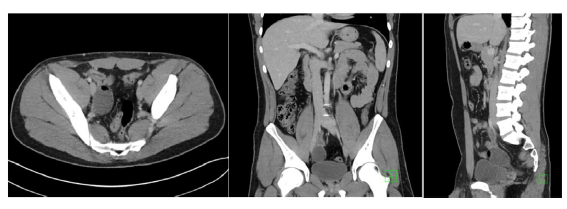
Given his past history of malignancy and clinical findings, a Computed Tomography (CT) scan was performed (Figure 1), which showed a fluid-filled mass extending from the small bowel into the pelvis consistent with a Meckel’s diverticulum.
Figure 1: Intravenous-enhanced CT abdomen and pelvis performed in the portal venous phase.
Selected axial (i), coronal (ii) and sagittal (iii) images show a tubular, blind-ending fluid and gas-filled digestive structure arising from the distal ileum, with mild associated inflammatory change and small volume of peritoneal free fluid.
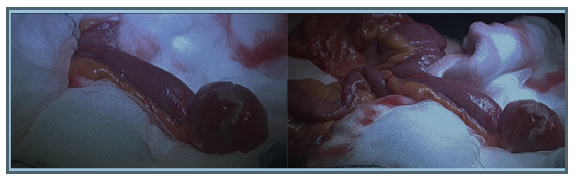
Diagnostic laparoscopy showed a MD 15.24cm in length (Figure2), which was excised using a laparoscopic surgical stapling device (Endo GIATM). The specimen was sent for histological analysis.The patient made a slow recovery jaded by a post-operative ileus which resolved conservatively and he was subsequently discharged home.
Histological findings indicated small intestinal type villous mucosa with no evidence of gastric or pancreatic mucosa, with severe inflammation and ulceration in keeping with Meckel’s diverticulitis. No evidence of neoplasia was found.
Figure 2: Laparoscopic views of giant MD with distal inflammation and oedematous changes.
DISCUSSION
MD is the only true diverticulum of the GI tract, comprising all three layers of the bowel wall and results from the incomplete atrophy of the vitelline duct. While 90% of MD are between 1 cm to 10 cm in length,‘giant MD’ exceeding 5 cm are rare [17], with the largest recorded nearly a century ago measuring over 100 cm [18].
Although the majority of MD remain asymptomatic and are discovered incidentally, the lifetime incidence of symptomatic presentation remains between 4% to 9% [4,9,19], compared to 7% to 8% in appendicitis [16].
Intestinal obstruction is the most common complication of symptomatic MD, followed by haemorrhage from peptic ulceration with heterotopic mucosa, and diverticulitis with or without perforation, as in this case [20], accounting for 90% of symptomatic MD [19]. Obstruction arises from multiple aetiologies, including intussusceptions, whereby the MD is the lead point; mechanical volvulus around a persistent fibrous band attaching the MD to the umbilicus, or axial twisting around a narrow base; diverticular stricture; Littre’s herniation, and inflammatory adhesions [2]. Rarer complications include umbilical abnormalities such as fistulation [21] and Meckelian cancers, generally diagnosed after 60 years of age [21,22].
Risk factors for developing complications are well established.Male sex, age under 50 years, length greater than 2 cm, and heterotopic presence of gastric or pancreatic mucosa increase the risk of complicated MD [23]. Presence of two, three (seen here) or four crieteria increases the symptomatic prevalence to 25%, 42% and 70% respectively [23]. Nonetheless, a high index of suspicion is necessary for prompt diagnosis and treatment [24], with only 4% of MD identified pre-operatively either clinically or radiologically [25]. The presence of heterotopic mucosa (gastric and pancreatic tissue accounting for 97 %) is the most significant factor for determining the need for surgical intervention, and is closely correlated with haemorrhage. Symptomatic MD decreases with age (10, 13, 26-30), with over half of all children with MD requiring surgery under 5 years [31]. Interestingly, Negrea V et al. [32] found a higher nerve fiber density in the walls of the Meckel's lined with intestinal mucosa, as seen in this case, compared to areas lined with ectopic gastric mucosa and the walls of the ileum. An inverse correlation exists between nerve fibre density and age, with higher nerve fibre density resulting in more intense local peristalsis, which may explain the observed predisposition to intussusception [32].
Giant MD has a higher incidence of obstruction [19,33]. Moreover, diverticulitis, torsion and volvulus are more common complications in longer MDs with a narrow base, while short MDs with a wider base stand at higher risk of intussusception [34]. Thus, an elongated variant with a narrow neck is more likely to result in torsion and diverticulitis, as seen in this case, whereas a short, wide-base diverticula may promote foreign body entrapment [15]. To the author’s knowledge, this is one of the longest MD reported in the literature, with an abnormal presentation with near normal inflammatory markers.
Definitive management remains surgical excision of the MD with or without resection of adjacent small bowel, the latter preferred in the presence of severe inflammation [34]. Laparoscopic procedures have been shown to be safe and confer no worse outcomes or complications than open surgery, through single or 3 trocars, intra-peritoneally or exteriorization [35,36]. Debate exists whether silent MD should be resected when incidentally discovered. Recent reviews by Zani et al. [9] and Soltero et al. [19] found that resection of silent MD conferred a significantly higher post-operative complication rate than leaving in situ. Zani et al. found a 5.3% risk of postoperative complications after prophylactic resection and a 1.3% risk of developing symptoms after leaving it in situ. They also found no long-term complications associated with leaving the Meckel's in situ when reviewing articles that reported follow-up on patients with silent Meckel's left in situ, and estimated that more than 750 silent MD would have to be resected in order to preserve one life.
CONCLUSIONS
This case demonstrates the highly variable presentation of giant Meckel’s diverticulitis, conferring considerable diagnostic and, given its considerable length, operative challenges. A diagnosis of MD should always be considered in the differential of an acute abdomen presenting with right iliac fossa pain, and multi-modality investigations including CT are recommended.
Acknowledgements
The authors are indebted to Dr Benjamin Butler, specialty registrar in radiology, for his provision and reporting of the images in Figure 1.
REFERENCES
- Uppal K, Tubbs RS, Matusz P, Shaffer K, Loukas M (2011) Meckel's diverticulum: a review. Clin Anat 24(4): 416-422.
- Hansen CC, Søreide K (2018) Systematic review of epidemiology, presentation, and management of Meckel's diverticulum in the 21st century. Medicine (Baltimore) 97(35): e12154.
- Sagar J, Kumar V, Shah DK (2006) Meckel's diverticulum: a systematic review. J R Soc Med 99(10): 501-505.
- Elsayes KM, Menias CO, Harvin HJ, Francis IR (2007) Imaging manifestations of Meckel's diverticulum. AJR Am J Roentgenol 189(1): 81-88.
- Tenreiro N, Moreira H, Silva S, Madureira L, Gaspar J, Oliveira A (2015) Unusual presentation of a Meckel’s diverticulum: A case report. Int J Surg Case Rep 16: 48-51.
- Palanivelu C, Rangarajan M, Senthilkumar R, Madankumar MV, Kavalakat AJ (2008) Laparoscopic management of symptomatic Meckel's diverticula: a simple tangential stapler excision. JSLS Society of 12(1): 66-70
- Freeman HJ (2001) Meckel's diverticulum in Crohn's disease. Can J Gastroenterol 15(5): 308-311.
- Tauro LF, George C, Rao BS, Martis JJ, Menezes LT, Shenoy HD (2010) Asymptomatic Meckel’s diverticulum in adults: is diverticulectomy indicated? Saudi J Gastroenterol 16(3):198-202
- Zani A, Eaton S, Rees CM, Pierro A (2008) Incidentally detected Meckel diverticulum: to resect or not to resect? Ann Surg 247(2): 276-281.
- Aarnio P, Salonen IS (2000) Abdominal disorders arising from 71 Meckel's diverticulum. Ann Chir Gynaecol 89(4): 281-284.
- Sancar S, Demirci H, Sayan A, Ar?kan A, Candar A (2015) Meckel’s diverticulum: ten years’ experience. Ulus Cerrahi Derg 31(2): 65-67.
- Shalaby RY, Soliman SM, Fawy M, Samaha A (2005) Laparoscopic management of Meckel's diverticulum in children. J Pediatr Surg 40(3): 562-567.
- Ueberrueck T, Meyer L, Koch A, Hinkel M, Kube R, Gastinger I (2005) The significance of Meckel’s diverticulum in appendicitis—a retrospective analysis of 233 cases. World J Surg 29(4): 455-458.
- Akbulut S, Yagmur Y (2014) Giant Meckel’s diverticulum: An exceptional cause of intestinal obstruction. World J Gastrointest Surg 6(3): 47-50.
- Tan YM, Zheng ZX (2005) Recurrent torsion of a giant Meckel’s diverticulum. Dig Dis Sci 50(7): 1285-1287.
- Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT (2015) Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet 386(10000): 1278-1287.
- Torii Y, Hisatsune I, Imamura K, Morita K, Kumagaya N, Nakata H (1989) Giant Meckel diverticulum containing enteroliths diagnosed by computed tomography and sonography. Gastrointest Radiol 14(1): 167-169.
- Tisdall FF (1928) An unusual Meckel's diverticulum as a cause of intestinal hemorrhage. Am J Dis Child 36(6): 1218-1223.
- Soltero MJ, Bill AH (1976) The natural history of Meckel's diverticulum and its relation to incidental removal: a study of 202 cases of diseased Meckel's diverticulum found in King County, Washington, over a fifteen year period. Am J Surg 132(2): 168-173.
- Levy AD, Hobbs CM (2004) From the archives of the AFIP: Meckel diverticulum: radiologic features with pathologic correlation. Radiographics 24(2): 565-587.
- Durakbasa CU, Okur H, Mutus HM, Bas A, Ozen MA, Sehiralti V, et al. (2010) Symptomatic omphalomesenteric duct remnants in children. Pediatr Int 52(3): 480-484.
- Thirunavukarasu P, Sathaiah M, Sukumar S, Bartels CJ, Zeh III H, Lee KK, et al. (2011) Meckel's diverticulum - a high-risk region for malignancy in the ileum: insights from a population-based epidemiological study and implications in surgical management. Ann Surg 253(2): 223-230
- Mackey WC, Dineen P (1983) A fifty year experience with Meckel's diverticulum. Surg Gynecol Obstet 156(1): 56-64.
- Rivas H, Cacchione RN, Allen JW (2003) Laparoscopic management of Meckel's diverticulum in adults. Surg Endosc 17(4): 620-622.
- Lüdtke FE, Mende V, Köhler H, Lepsien G (1989) Incidence and frequency or complications and management of Meckel's diverticulum. Surg Gynecol Obstet 169(6): 537-542.
- Park JJ, Wolff BG, Tollefson MK, Walsh EE, Larson DR (2005) Meckel diverticulum: the Mayo Clinic experience with 1476 patients (1950–2002). Ann Surg 241(3): 529-533.
- Feller AA, Movson J, Shah SA (2003) Meckel diverticulum: a geriatric disease masquerading as common gastrointestinal tract disorders. Arch Intern Med 163(17): 2093-2096.
- Ero AP, Martinez-Barba E, Canteras M, Rodriguez JM, Castellanos G, Parrilla P (2002) Surgical management and complications of Meckel's diverticulum in 90 patients. Eur J Surg 168(1): 8-12.
- Varcoe RL, Wong SW, Taylor CF, Newstead GL (2004) Diverticulectomy is inadequate treatment for short Meckel's diverticulum with heterotopic mucosa. ANZ J Surg 74(10): 869-872.
- Lohsiriwat V, Sirivech T, Laohapensang M, Pongpaibul A (2014) Comparative study on the characteristics of Meckel's diverticulum removal from asymptomatic and symptomatic patients: 18-year experience from Thailand's largest university hospital. J Med Assoc Thai 97(5): 506-512.
- Alemayehu H, Hall M, Desai AA, Peter SD, Snyder CL (2014) Demographic disparities of children presenting with symptomatic Meckel’s diverticulum in children’s hospitals. Pediatr Surg Int 30(6): 649-653.
- Negrea V, Gheban D (2012) Nervous structure of Meckel’s diverticulum in children. Rom J Morphol Embryol 53(3): 573-576.
- Cartanese C, Petitti T, Marinelli E, Pignatelli A, Martignetti D, Zuccarino M, et al. (2011) Intestinal obstruction caused by torsed gangrenous Meckel’s diverticulum encircling terminal ileum. World J Gastrointest Surg 3(7): 106-109.
- Caiazzo P, Albano M, Del Vecchio G, Calbi F, Loffredo A, Pastore M, et al. (2011) Intestinal obstruction by giant Meckel's diverticulum. Case report. Il G Chir 32(11-12): 491-494.
- Ding Y, Zhou Y, Ji Z, Zhang J, Wang Q (2012) Laparoscopic management of perforated Meckel's diverticulum in adults. Int J Med Sci 9(3): 243-247
- Clark JM, Koontz CS, Smith LA, Kelley JE (2008) Video-assisted transumbilical Meckel's diverticulectomy in children. Am Surg 74(4): 327- 329.
Copyright: Zaman S, et al. ©2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Zaman S (2019). An Atypical Presentation of Giant Meckel’s Diverticulitis: A Case Report. Gastro Res 1(1): 1.
 Abstract
Abstract  PDF
PDF